Experiment 9 Extras
pH meter
Thermometer(-10-100C)
UV light
-Longwave at 365nm
-Shortwave at
254nm
Blender
Mortar and pestle
chopping board and
knife
Strainer
Stopwatch
Juicer
Oven
mitt
Experiment 9 Extras
Filter paper
-(7 and 12.5cm)
Scissors
Ice
Microwave
-Wattage labelled on microwave
door
Beakers (various sizes)
Volumetric pipets (5, 10, 20, 50mL)
Serological pipet
10mL
Volumetric flasks
-(50, 100, 200, 250, 500mL, 1L)
Ovens
-Set at different
temperatures
between 60C to 200C (20C increments)

Top Loading Balance
The top loading balance is used to weigh a sample where a high degree of accuracy is not needed.
Accuracy: ± 0.1g or ± 0.01g.
Analytical Balance
The analytical balance is used for analytical work and weighs samples very accurately.
Accuracy: ± 0.0001g.
Fume Hood
A fume hood (sometimes called a fume cupboard or fume closet) is a type of local ventilation device that is designed to limit exposure to hazardous or toxic fumes, vapours or dusts. It has a sash window that can be pulled down to limit exposure more.

Hot Plate
Used to heat solutions and aid in dissolving solids.

Magnetic Stirrer with Stir Bar
Used to mix solutions. In this course the magnetic stirrer is used to continuously mix solutions during a titration, to ensure uniformity.

Magnetic Stir Bar
Used to mix solutions. In this course the stir bar is used to continuously mix solutions during a titration and kinetic traces, to ensure uniformity.

Oven
Used for heating and drying.

Spectrophotometer (Microlab)
An instrument that measures the intensity of light absorbed by a sample. Certain wavelengths of light are absorbed by the molecules in the sample, whereas others are transmitted. There is a black cap that covers the sample while it is being tested to block ambient light. Vials used to hold the samples should be cleaned with a Kimwipe before being put into the spectrophotometer to avoid the oil from fingers distorting the light beam.

Oven Mitt/Glove
Used to protect hands from hot items in the lab.

Ceramic Tile
For resting hot objects on to protect the bench tops. Can also be placed under an Erlenmeyer flask during titrations to make it easier to determine the end point colour.

Ice Bath
Tub containing ice to cool down solutions.

Funnel (plastic)
Used for quantitative transfer of solutions.
Should always be removed after transferring
a
solution.
A drop of solution may be stuck in the funnel and can drop down at any time. This would add
volume to a burette that is unaccounted for or cause the solution to go above the calibration
mark in a
volumetric flask.

Funnel (glass)
Used for quantitative transfer of solutions.
Glass funnel will be used in experiment 10

Filter Paper
Used to separate solids from liquids by being placed in a funnel (glass or Buchner). A solution containing a precipitated solid is poured through the filter paper in the funnel, collecting the solid on the filter paper and allowing the liquid to pass through. Filter paper is manufactured with differing sized pores to collect different particle size.

Vials with Plastic Lids
Used to hold solutions to be tested in the Microlab spectrophotometer.
Never write with
marker on
vials and always wipe them with kimwipe before putting in MicroLab. Light passes through the
vial and if
any
obstruction is in the way it will give an inaccurate reading

Glass Stir Rod
Used for mixing solutions.
Always rinse back into beaker after mixing. Solid or solution
may be
stuck to the glass stir rod and must be rinsed back into the beaker. If this is not done, the
original
measured amount will be lower.
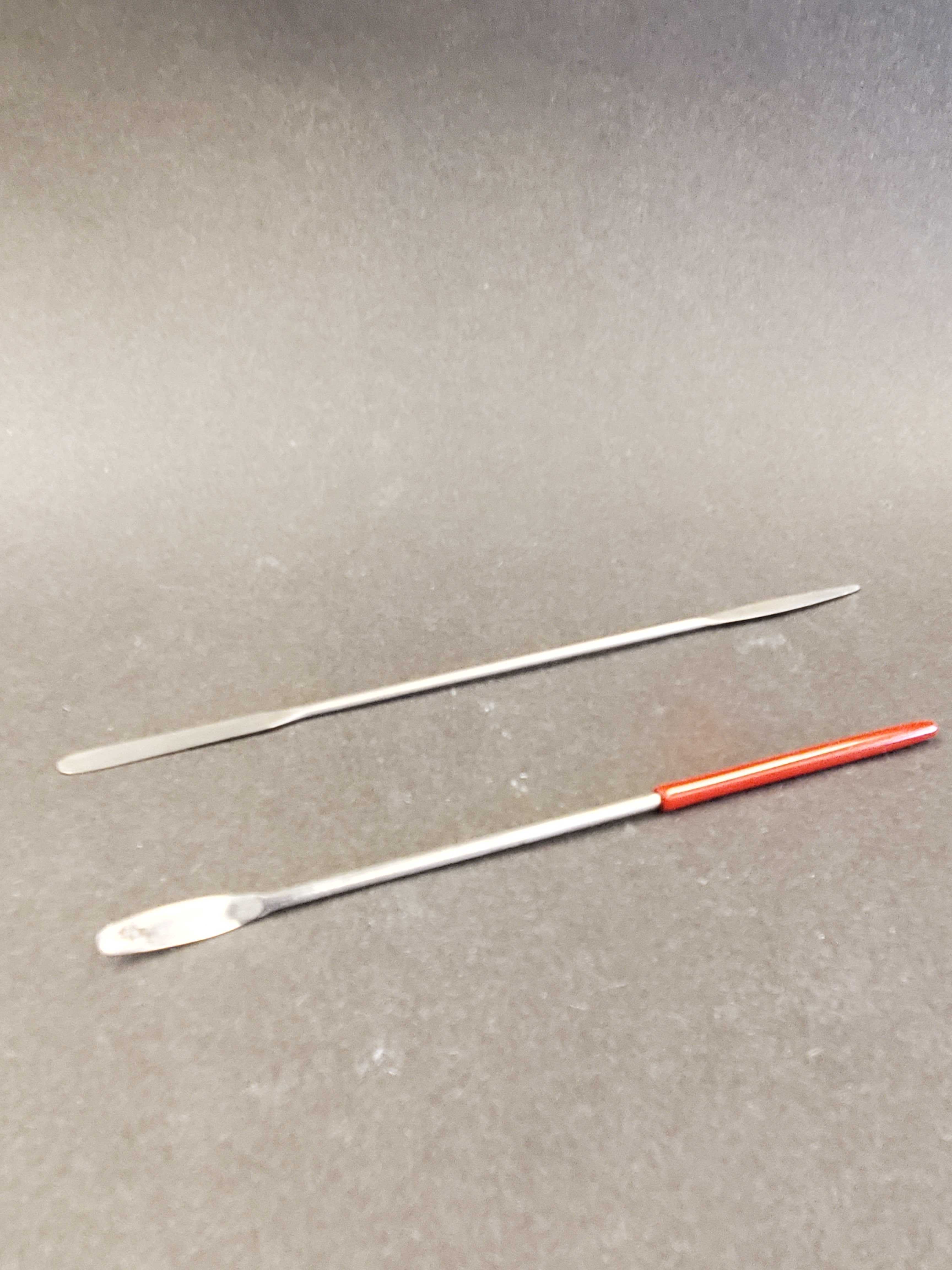
Spatula
Used to scoop out small amounts of solid chemicals for weighing.
Never use to mix
solutions.
Metal
may react with chemicals in solution

Watch Glass
Used for weighing products and to stop foreign objects from falling into a beaker during heating. The watch glass should be placed on a beaker with the curved side down (as in picture) so that any condensation drips back into the beaker or if any solution “bumps” it will drip back down into the beaker. Keep on top of beaker if cooling solution, so that debris cannot fall in solution adding unaccounted weight. Note: always place a watch glass on the bench with the curved side facing up to avoid debris on bench top can be transferred into beaker adding unaccounted weight.

Pasteur Pipet
Used to deliver small amounts of liquid, where the quantity does not need to be known. Can also be made out of plastic.

Weigh Boat
Used in weighing to hold the compound being weighed.

Water Bottle for Deionized Water
Pre-rinse glassware 3 times with deionized water. Do not use for washing at the end of lab. Please use hot tap water

Rotary Evaporator
Used to remove solvents from a sample by evaporation. A vacuum pump is used to lower the pressure which decreases the boiling point. A motorized rotator rotates the sample, increasing the surface area of the liquid inside and prevents the solvent from bumping. A water bath is maintained at a temperature suitable to the solvent being evaporated. The solvent begins to evaporate and the gaseous fumes will travel up in condenser. A coolant running through the condenser will allow the gaseous solvent to condense. Once done, the separation from solute is complete

Melting Point Apparatus
An electronic device equipped with a thermometer, a magnifying glass, and a heating block used to identify compounds based on the temperature it turns from a solid to a liquid.

Capillary Tube
A thin glass tube that has been sealed closed at one end and is used to hold the solid sample that is being analyzed

Capillary Tube Dropper
Used to pack a solid sample at the bottom of a capillary tube by dropping it through. Ensure the closed end is down

Vacuum Sublimation
A separation technique used to purify a solid chemical substance.

Drop Dispenser
Used to pack a solid sample at the bottom of a capillary tube by dropping it through. Ensure the closed end is down

pH Probe in pH 7 Buffer
Used to determine pH in a solution by taking electrochemical measurments between two electrodes contained within the probe. This prove is very fragile and expensive. Please use carefully

Test Tubes
Used for holding, heating, and storing chemicals. They are made of glass so they can withstand heat and the reaction can be easily viewed. The long neck controls the speed of escaping gasses during a reaction.

Kim Wipes
Used to clean vials before placing into the spectrophotometer to avoid the oil from fingers distorting the light beam.

Drop Counter
Has a sensor that counts the number of drops coming out of the drop dispenser. Ensure that drop dispenser is lined up with the drop counter.

Syringe
In this course the syringe will be used in the kinetics lab to measure a volume of bleach that will be dispensed into a vial of dye

Vacuum Outlet Adapter
Used for secondary drying after using the rotary evaporator



