
Top Loading Balance
The top loading balance is used to weigh a sample where a high degree of accuracy is not needed.
Accuracy: ± 0.1g or ± 0.01g.
Analytical Balance
The analytical balance is used for analytical work and weighs samples very accurately.
Accuracy: ± 0.0001g.
Bunsen Burner
Is a kind of gas burner used as laboratory equipment; it produces a single open gas flame, and is used for heating, sterilization, and combustion. In this course the Bunsen is used to perform flame tests to detect and confirm the presence of certain chemicals.

Chromatography Column
Used to separate chemicals into their various components. It comprises of a glass cylinder containing a mobile phase (water) and a stationary phase (silica gel), with a tube on the bottom where the separate components are drawn out using a syringe.

Fume Hood
A fume hood (sometimes called a fume cupboard or fume closet) is a type of local ventilation device that is designed to limit exposure to hazardous or toxic fumes, vapours or dusts. It has a sash window that can be pulled down to limit exposure more.

Hot Plate
Used to heat solutions and aid in dissolving solids.

Magnetic Stirrer with Stir Bar
Used to mix solutions. In this course the magnetic stirrer is used to continuously mix solutions during a titration, to ensure uniformity.

Oven
Used for heating and drying.

Spectrophotometer (Microlab)
An instrument that measures the intensity of light absorbed by a sample. Certain wavelengths of light are absorbed by the molecules in the sample, whereas others are transmitted. There is a black cap that covers the sample while it is being tested to block ambient light. Vials used to hold the samples should be cleaned with a Kimwipe before being put into the spectrophotometer to avoid the oil from fingers distorting the light beam.
.jpg)
Vacuum filtration apparatus (when using a crucible)
Vacuum is used to pull the liquid through the crucible and into the filtration flask below, leaving the compound under investigation, captured in the crucible. The black rubber adapter, makes the vacuum seal better. If you didn’t use the adapter, there would be no seal between the neck of the glass filtration flask and the glass crucible.
.jpg)
Vacuum filtration apparatus (when using a Buchner funnel)
Vacuum is used to pull the liquid through the Buchner funnel and filter paper and into the filtration flask below. Depending on the experiment being performed sometimes you are interested in the liquid collected in the flask and other times the compound collected on the filter paper. The grey rubber adapter, makes the vacuum seal better. If you didn’t use the adapter, there would be no seal between the neck of the glass filtration flask and the plastic Buchner funnel.

Cool Air Gun
Used for rapidly cooling down equipment and solutions.

Oven Mitt/Glove
Used to protect hands from hot items in the lab.

Ceramic Tile
For resting hot objects on to protect the bench tops. Can also be placed under an Erlenmeyer flask during titrations to make it easier to determine the end point colour.

Ice Bath
Tub containing ice to cool down solutions.

Funnel
Used for quantitative transfer of solutions.
Should always be removed after transferring
a
solution.
A drop of solution may be stuck in the funnel and can drop down at any time. This would add
volume to a burette that is unaccounted for or cause the solution to go above the calibration
mark in a
volumetric flask.

Petri Dish
In this course, the Petri dish is used to allow crystals to grow in.

Filter Paper
Used to separate solids from liquids by being placed in a funnel (glass or Buchner). A solution containing a precipitated solid is poured through the filter paper in the funnel, collecting the solid on the filter paper and allowing the liquid to pass through. Filter paper is manufactured with differing sized pores to collect different particle size.

Vials with Plastic Lids
Used to hold solutions to be tested in the Microlab spectrophotometer.
Never write with
marker on
vials and always wipe them with kimwipe before putting in MicroLab. Light passes through the
vial and if
any
obstruction is in the way it will give an inaccurate reading

Glass Stir Rod
Used for mixing solutions.
Always rinse back into beaker after mixing. Solid or solution
may be
stuck to the glass stir rod and must be rinsed back into the beaker. If this is not done, the
original
measured amount will be lower.
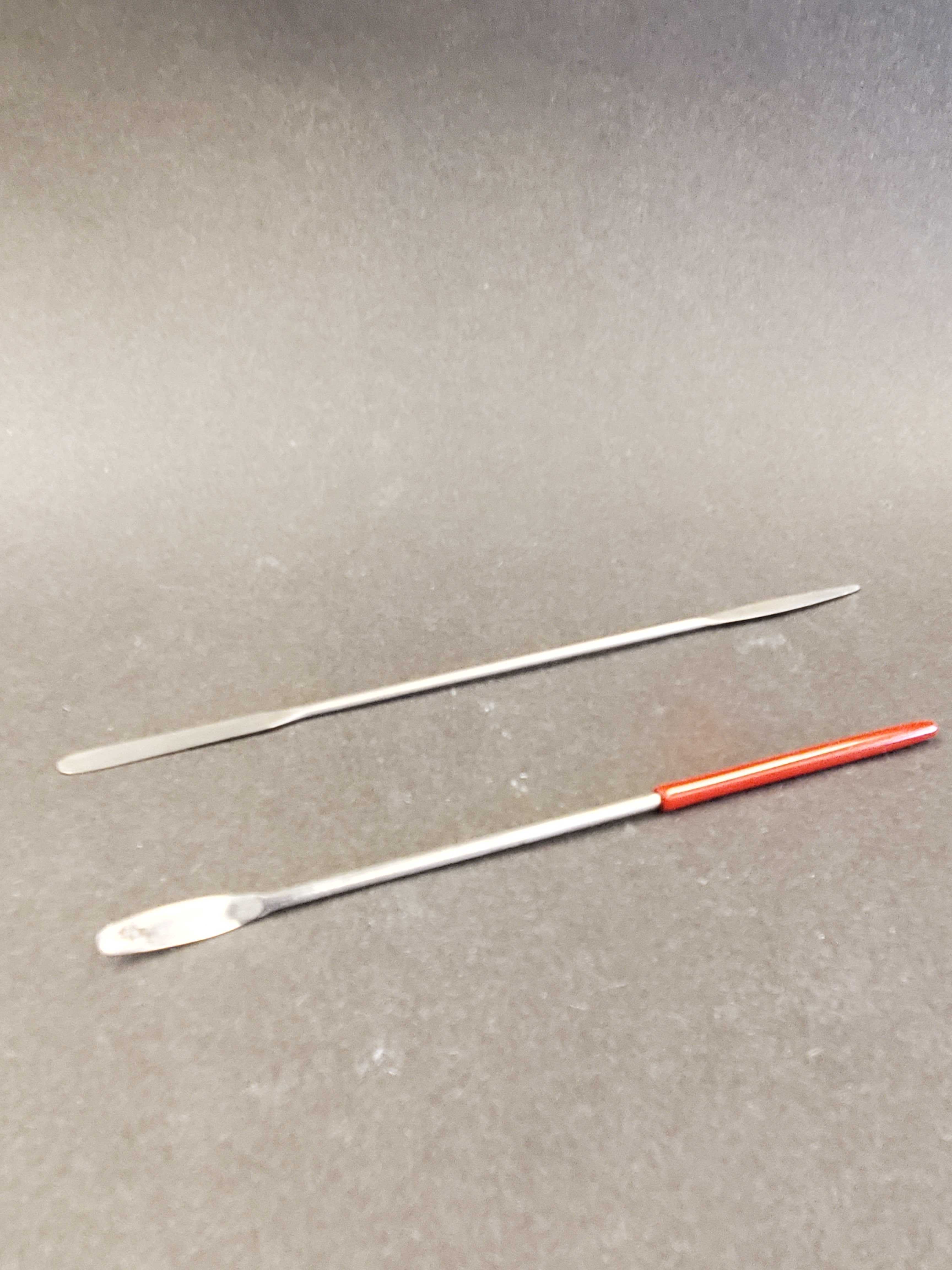
Spatula
Used to scoop out small amounts of solid chemicals for weighing.
Never use to mix
solutions.
Metal
may react with chemicals in solution

Watch Glass
Used for weighing products and to stop foreign objects from falling into a beaker during heating. The watch glass should be placed on a beaker with the curved side down (as in picture) so that any condensation drips back into the beaker or if any solution “bumps” it will drip back down into the beaker. Keep on top of beaker if cooling solution, so that debris cannot fall in solution adding unaccounted weight. Note: always place a watch glass on the bench with the curved side facing up to avoid debris on bench top can be transferred into beaker adding unaccounted weight.

Pasteur Pipet
Used to deliver small amounts of liquid, where the quantity does not need to be known. Can also be made out of plastic.

Syringe
Used to draw liquid through a chromato-graphy column.

Weigh Boat
Used in weighing to hold the compound being weighed.

Litmus Paper
The main use of litmus paper is to test whether a solution is acidic or basic. Red litmus paper turns blue under basic or alkaline conditions, with the colour change occurring over the pH of 8.3 at 25°C.

Water Bottle for Deionized Water
Pre-rinse glassware 3 times with deionized water. Do not use for washing at the end of lab. Please use hot tap water.

Test Tubes
Used for holding, heating, and storing chemicals. They are made of glass so they can withstand heat and the reaction can be easily viewed. The long neck controls the speed of escaping gasses during a reaction.

Nichrome Wire
Can withstand very high temperatures. Used to place compounds into the flame of a Bunsen burner when performing flame tests.

Aluminum Foil Lid
Used to cover beaker containing a crucible. Hole already poked to let out water vapour. Always
ensure
lid is
on when sitting on benchtop or cooling so that debris does not fall in and add weight
DO
NOT
THROW
AWAY. RETURN IN DESIGNATED BINS

Bottle for diluting NaOH
Used in the titration expiriment to dilute NaOH



