
| Learning Goal 15 |
|---|
| Explain how an acid's strength is related to its polarity and the strength of the H-X bond. |
How easily will a molecule containing an H-X bond break, resulting in proton donation?
1. A substance will only donate a proton if the H-X bond is polar.

2. The strength of the H-X bond affects the molecule's ability to donate its protons.
| Generally, the stronger the acids, the weaker and more polar their H-X bonds. Hence, strong acids give up protons more easily! |
|---|
A hydride is a compound formed between hydrogen and any other element.
In each horizontal row of the periodic table, the most basic hydrides are on the left and the most acidic hydrides are on the right. Note that the left forms metal hydrides and the right forms non-metal hydrides. Metal hydrides are usually basic in water while non-metal hydrides are often acidic in water.
NaH = basic hydride:
HCl = acidic hydride:
In each vertical row of the periodic table, acidity usually increases with increasing atomic number because the valence (bonding) electrons are farther and farther away from the nucleus and less strongly attracted to the positive nucleus. Thus, the bonds formed by these electrons decrease in strength, making the bond easier to break and release protons.

| Learning Goal 16 |
|---|


Acid strength increases with :
1. increasing electronegativity of the central atom: The more electronegative the central atom, the more electron drift occurs, polarizing the O-H bond which makes it easier for the molecule to dissociate.
2. increasing oxidation number of the central atom: The higher the oxidation state of the central atom, the greater the number of oxygens attached to the central atom. Oxygen is a highly electronegative element, contributing to greater electron drift away from the O-H bond.
An oxyacid is an acid in which (OH) groups (and sometimes additional oxygen atoms) are bound to a central atom. Oxyacids are a special subset of species which have a central atom "Y" which is bonded to an hydroxyl (OH) group and may or may not be bonded to other additional atoms.
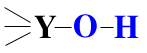
If "Y" has a low electronegativity (usually a metal) it will NOT attract the electron pair it shares with the O.
Therefore the O will be negatively charged: it will not attract the electron pair it shares with the H, and the O-H bond will NOT be strongly polarized. However, the Y-O bond will be highly polar and easily broken, releasing