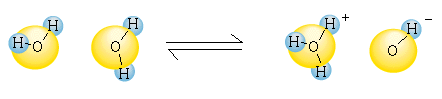The Dissociation of Water
| Learning Goal 7
|
|---|
| Explain what is meant by the autoionization of water, and
write the ion-product-constant expression for this process.
|
can act as both a proton donor and acceptor for
itself. A proton can be transferred from one water molecule to
another, resulting in the formation of one hyroxide ion
() and one hydronium ion ().
This is called the autoionization or
dissociation of water. This equilibrium can also be
expressed as:
In the above equilibrium, water acts as both an acid and a base. The ability of a species to act as either an acid or a base is known as amphoterism.
The concentrations of and and is called the ion-product
constant for water. At 25oC, KW is equal to
10-14.
The ion-product constant always remains constant at
equilibrium (as the name implies). Consequently, if the concentration
of either H+ or OH- rises, then the
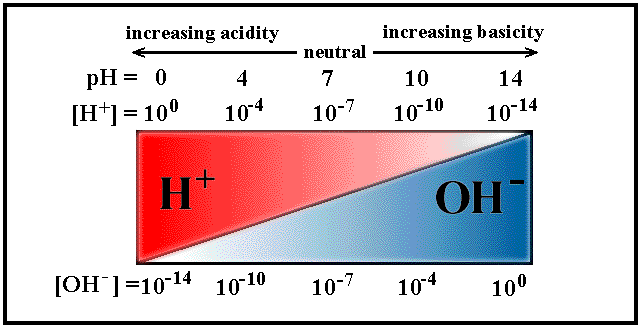
other must fall to compensate. In acidic solutions, [H+] >
[OH-], and in basic solutions [H+] <
[OH-].
The pH Scale
| Learning Goal 8
|
|---|
| Define pH; calculate pH from a knowledge of
[H+] or [OH-], and perform the reverse
operation.
|
Rather than expressing [H+] as some very small number,
it is often more convenient to describe in terms of
pH, defined as:
pH = - log [H+]
For example, a neutral solution at 25oC contains equal concentrations of H+ ions and OH- ions, where [H+] =
10-7M. Thus, the pH of the solution is obtained by:
pH = - log 10-7 = 7
From this it is obvious that at 25oC:
| pH < 7
| solution is acidic
|
| pH = 7
| solution is neutral
|
| pH > 7
| solution is basic
|
Notice that the pH of a solution measures the concentration of dissociated protons, and not the total concentration of acid in a solution.
The negative log scale is useful for measuring other minute
quantities, for example to measure [OH-]:
pOH = - log [OH-]
Knowing this, we obtain the following useful expression:
pH + pOH = -log KW = 14.00
The Relationship Between pH and pOH
| pH
| pOH
| [H+] mol/L
| [OH-] mol/L
|
| 0
| 14
| 1.0
| 10-14
| 2
| 12
| 0.01
| 10-12
|
| 4
| 10
| 0.0001
| 10-10
|
| 6
| 8
| 10-6
| 10-8
|
| 8
| 6
| 10-8
| 10-6
|
| 10
| 4
| 10-10
| 0.0001
|
| 12
| 2
| 10-12
| 0.01
|
| 14
| 0
| 10-14
| 1.0
| |