8. Kw is not constant with respect to temperature, but rather decreases as the temperature of the water decreases. At 10oC, = 3.0 x . What is the pH of pure water at 10oC?
First, we need to substitute into the Kw expression. Given
H2O (l) 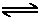 H+ (aq) + OH- (aq) ,
H+ (aq) + OH- (aq) ,
what is the relationship between [ H+ ] , [ OH- ] and [ H2O ] ?



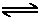 H+ (aq) + OH- (aq) ,
H+ (aq) + OH- (aq) ,
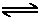 H+ (aq) + OH- (aq) ,
H+ (aq) + OH- (aq) ,


