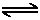 +
+ Most substances that are acidic in water are actually weak acids. Because weak acids dissociate only partially in aqueous solution, an equilibrium is formed between the acid and its ions. The ionization equilibrium is given by:
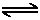 +
+

However, keep in mind that the "Before Dissociation" state never actually exists in solution--the solution is always at equilibrium. The left hand state is theoretical.
The equilibrium constant is then:
Click here or on the ![]() button on the menu bar for a table of Ka values for common weak acids.
button on the menu bar for a table of Ka values for common weak acids.
The smaller the value for
POINT OF EMPHASIS: Do not confuse a weak acid with a dilute acid. A weak acid has a small
| Learning Goal 10 |
|---|
| Calculate the pH for a weak acid solution in water, given the acid concentration and |
STEP 1: Write the ionization equilibrium for acetic acid:
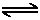 H+ (aq) + C2H3O2- (aq)
H+ (aq) + C2H3O2- (aq)STEP 2: Create an I.C.E. grid, and determine the concentration from information provided in the problem:
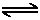 H+ (aq) + C2H3O2- (aq)
H+ (aq) + C2H3O2- (aq)| INITIAL
| CHANGE
| EQUILIBRIUM
| |
|---|
STEP 3: Substitute the equilibrium concentrations into the equilibrium constant expression:
The equilibrium constant expression is:
This equation has only one unknown and can be solved using the quadratic formula. However, we can make things easier:
STEP 4: Since the value of
and STEP 5: Now we find pH:
| Learning Goal 11 |
|---|
| Calculate the percentage ionization for an acid or a base, knowing its concentration in solution, and the value of |
Percent ionization or percent dissociation is defined as:

The percent dissociation of an acid varies with the concentration of the acid. The more dilute an acid is, the greater the percent ionization. Why?
Let's look at the equilibrium of a weak acid again:
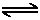
From Le Chatelier's Principle, adding water to the equilibrium would cause the equilibrium to shift to the right. A shift to the right implies that more acid would be in dissociated form, and thus the percent ionization increases accordingly.
STEP 1: Ionization equation:
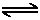 H+ (aq) + F- (aq)
H+ (aq) + F- (aq)STEP 2: I.C.E. grid:
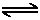 H+ (aq) + F- (aq)
H+ (aq) + F- (aq)
| HF | |||
|---|---|---|---|
| INITIAL | |||
| CHANGE | |||
| EQUILIBRIUM |
However,
which is greater than 5% of the initial concentration of the acid, so x is not x << 0.10M!
STEP 5: Use quadratic:
Therefore,


